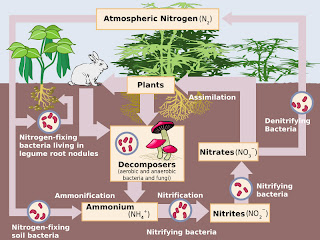
 |
| The Nitrogen Cycle - taken from Wikipedia |
Modern industrial agriculture does away with all this. Instead of a diverse ecosystem providing these plants with food, we supply the bulk of the nutrients by manufacturing artificial fertiliser.
Artificially fixing nitrogen is a complicated process. We first take hydrogen from fossil fuels. Then we combine it with nitrogen to make ammonia.
 |
| The Haber Process - creating ammonia from nitrogen and hydrogen |
Growing food becomes a partially linear, rather than completely cyclical process. Nutrients are dug up from mines and transported to be manufactured into fertiliser. The fertiliser is used on our crops, we eat the food and dump the scraps in landfill, and pump our sewerage into the ocean. There are also other points where nutrients leave the system along the way, such as topsoil lost through irrigation and erosion. In one end, out the other so to speak.
I'll make a lego analogy: Let's say I buy a box of lego and build a new object. I play with the thing until it breaks apart. Instead of rebuilding the object out of the bits I've already got, I push the pieces into a pile and buy another box.
 |
| My horribly oversimplified lego analogy of the biogeochemical cycle |
 |
| My horribly oversimplified lego analogy of how artificial fertiliser is used in industrial agriculture |
Plants also need phosphorus. It's mined mostly in places like Africa and the Middle East. And we're going to run out of it one day.
In this short article, I've only touched on 2 ingredients of fertiliser which are necessary for modern industrial agriculture. One of them is running out, and they both require a LOT of fossil fuel... which is also running out.
Even if we had infinite fossil fuel and phosphorus to dig up, we could still have some big problems in the future if, for example, conflict cut us off from cheap oil for an extended period. Our agriculture relies completely on a globally interdependent system. This situation seems a little precarious to me.
No comments:
Post a Comment